CE Marking Consultants For Medical Device
CE Marking Consultants will create a medical device technical file showing that devices, instruments, disposables, software, machinery, and home health care devices meet all of the EU Medical Device Regulation standards before applying the CE Mark. The manufacturer/importer must establish the CE marking logo on the medical device. The logo must be visible on both the primary and secondary packaging of the device.
Over the last four years, we have spent a significant amount of time and money building our in-house MDR CE Marking consultants team to make MEDDEVICECORP Company the best consultancy service provider.
By doing business remotely in the cloud, we can provide multidisciplinary insights into Risk Analysis, Biological Evaluation, Clinical Follow-up, and Clinical Evaluation whilst minimizing travel and lodging costs. We represent our global and local clients with the same intense emphasis on quality suitable for early CE marking, thanks to our organization’s common, clear strategy and structure.
Before the final implementation of the EU MDR (2017/745), all of the necessary and inevitable improvements are unknown. This will prove to be a time-consuming and difficult process for all medical device manufacturers. As a result, it is recommended that you stay informed about upcoming changes and their implications for your system and obtain expert technical advice from the MDR CE Marking consultants team.
Consult with us for Drug Device Combination (DDC) Products with us for fast NBOp Letter.
Steps to Obtain and Maintain MDR CE Marking:
- Classification and Conformity Assessment: Manufacturers must classify their devices based on risk and subject them to the appropriate conformity assessment procedure. This involves identifying the relevant standards, conducting testing, and producing technical documentation.
- Appointing an EU Representative: Manufacturers located outside the EU must appoint an European Authorized Representative within the EU to ensure compliance with MDR regulations.
- Technical Documentation: Manufacturers need to create comprehensive technical documentation that outlines the design, development, and manufacturing processes of their devices. This documentation serves as evidence of compliance and should be readily accessible for regulatory authorities.
- Clinical Evaluation and Post-Market Surveillance: Devices must undergo a thorough clinical evaluation to demonstrate their safety and performance. Post-market surveillance activities are also crucial to monitor device performance once it’s on the market.
- Declaration of Conformity: Manufacturers must issue a Declaration of Conformity, declaring that their device complies with the MDR’s requirements. This declaration is a prerequisite for affixing the CE mark.
- Affixing the CE Mark: Once all prerequisites are met, manufacturers can affix the CE mark to their devices, signaling compliance with MDR standards.
- Continual Compliance: MDR CE marking is not a one-time achievement; manufacturers must maintain compliance through regular monitoring, updating technical documentation, and addressing any changes in regulations or device design.
MDR CE marking is the hallmark of quality and safety in the EU medical device market. It represents a commitment to patient well-being and regulatory compliance. Manufacturers aiming to thrive in this landscape must embark on a journey of meticulous planning, testing, documentation, and ongoing compliance efforts.
With the CE marking, the EU seeks to ensure that medical devices entering its market adhere to the highest standards, fostering a safe and reliable healthcare environment for all stakeholders involved.
The overall objective of the CE Marking has been to ensure a high level of protection of human health and safety and to provide a framework that is Conducive to the Innovation and competitiveness of the European medical device market.
The manufacturer certifies that their product complies with the GSPR of the applicable European Regulation by applying the CE Mark. Once a product has received the CE Marking, it can travel freely within the European Union and the European Free Trade Association (EFTA) (EU). Additionally, it means that non-conforming goods may be withdrawn by customs and other enforcement agencies.
The Medical Devices Regulation (MDR) has had a significant impact on the CE marking process for medical devices within the European Union (EU). It introduces stricter requirements and regulations to ensure the safety and effectiveness of medical devices in the market.
One of the key changes brought about by MDR is the increased emphasis on clinical evidence. Manufacturers are now required to provide more robust and extensive clinical data to support the safety and performance claims of their devices. This includes conducting more rigorous clinical evaluations, performance studies, and post-market surveillance activities.
Additionally, MDR introduces a new classification system for medical devices, which affects the conformity assessment process and the involvement of notified bodies. Manufacturers must carefully assess the classification of their devices and adhere to the corresponding conformity assessment procedures.
Overall, understanding the impact of MDR on CE marking is crucial for manufacturers to ensure compliance with the updated regulations, navigate the complexities of the process, and successfully bring their medical devices to the European market.
MDR CE Marking Common Challenges
MDR CE marking presents several challenges for medical device manufacturers as they navigate the updated regulations. One common challenge is obtaining sufficient clinical evidence to meet the increased requirements. Manufacturers may face difficulties in conducting clinical evaluations, especially for novel or high-risk devices.
Overcoming this challenge involves careful planning and collaboration with experts to design and execute well-controlled studies, collect reliable data, and demonstrate the safety and effectiveness of the device.
Another challenge is managing the transition from the previous Medical Devices Directive (MDD) to MDR. Manufacturers must understand the new classification system, updated conformity assessment procedures, and documentation requirements. Adapting to these changes may require revisiting existing technical files and conducting gap analyses to identify areas that need revision or additional documentation.
Keeping up with the rapidly evolving regulatory landscape and staying updated with MDR guidance documents is another challenge. Manufacturers need to invest time and resources in monitoring regulatory changes, attending relevant workshops or conferences, and collaborating with CE Marking consultants who specialize in MDR compliance.
Additionally, compliance with the MDR may require additional resources and investments in terms of clinical studies, post-market surveillance systems, and documentation updates. Manufacturers need to assess their capabilities and allocate sufficient resources to meet the increased requirements.
To overcome these challenges, manufacturers can engage the services of experienced CE Marking consultants who can provide guidance on the regulations, help with clinical evaluation planning and study design, assist with documentation preparation, and offer expertise in navigating the complexities of the MDR CE marking process.
Close collaboration with notified bodies, involvement of cross-functional teams, and continuous monitoring of regulatory updates can also help manufacturers successfully overcome the challenges associated with medical device CE marking and ensure compliance with the regulations.
MDR CE Marking Approval Process
Before being sold in the European Economic Area, medical devices must be CE-marked. C The flow chart below answers many of the questions.
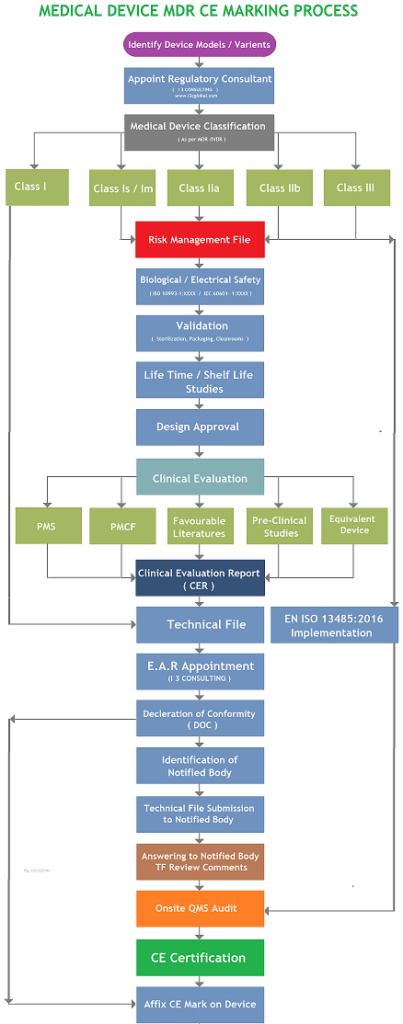
EU MDR 2017/ 745 CE Marking & Notified Body
EU MDR 2017/ 745 is the new regulation that makes its way after negotiations between the European Commission, European Parliament, and the European Council. New medical device regulation replaces the medical device Directives (93/42/EEC) and the Active Implantable Devices Directive (90/385/EEC).
The background work for the EU MDR by the European Commission dates back to 2012 when a plan to restructure the Regulatory Framework and revise the existing regulations was laid down. The new regulation was first read out in the Parliament in 2014, and the final text was available in June 2016. After the current translation works into the official European Languages, it is expected that the MDR will be available for application by May 2017.
EU MDR 2017/ 745 Medical Device Notified Bodies are authorized to issue CE marking by verifying the submitted technical documentation to prove the safety and performance of the device and to check its compliance with EU safety, health, and environmental regulations.
Major Changes and Medical Device Definition
- Increased accountability of European Authorized Representatives
- Stringent Clinical Evaluation
- More Technical Documentation Requirements
- EUDAMED Database
- Implementation of UID
- New classification method
Medical Device definition by MDR 2017/745, article 2
- General use medical devices (Sterile and Non-Sterile)
- Active Medical Devices
- Non-Active medical device
- Implantable Medical Devices
- Medical equipment and machinery
- Home Healthcare Devices
- Software as Medical Devices (SAMD)
- Active Implantable Medical Devices


Our Social Activities