FDA 510k submission Process For Medical Device
- Anyone who wants to sell a medical device in the United States of America is required to make a FDA 510k submission at least 90 days before offering the device for sale, even though it may have been under development or clinical investigation before that date.
- A new FDA 510k submission is required for changes or modifications to an existing medical device. The amendments could significantly affect the safety or effectiveness of the device, or the device is to be marketed for a new or different indication for use.
- Change in the intended use for a device that you already have in commercial distribution. (Already cleared 510k submission process)
- If there is a change or modification of a legally marketed device, that change could significantly affect its safety or effectiveness.
US FDA 510k Number
US FDA 510k number is a premarket notification number provided by the FDA after a manufacturer or specification developer has demonstrated that the medical device is substantially equivalent in performance and safety to other devices already existing on the market.
An FDA 510k is not a premarket approval, neither establishment registration nor device listing. In three situations, an FDA 510k is usually needed.
- The first is if it’s the first time bringing a new device to the market.
- The second is to change the indications for the use of a previously approved product,
- The third is to make significant changes to a previously approved device.
In these circumstances, you need 510k approval before you can register, list, or begin marketing.
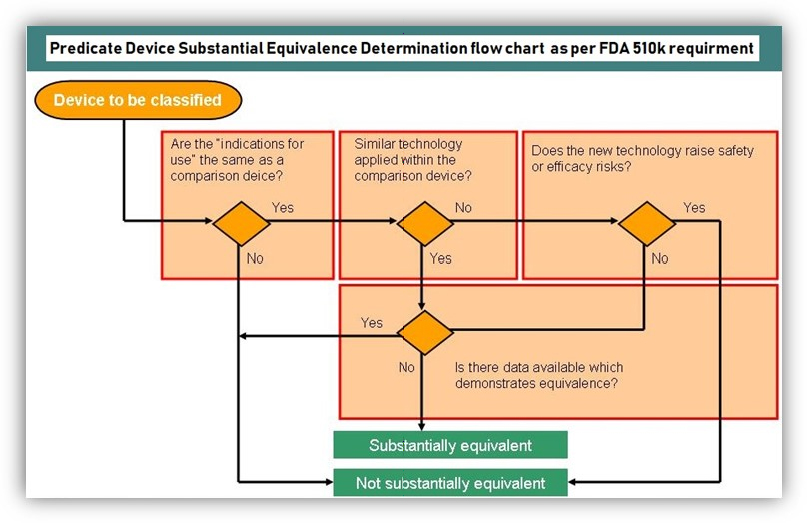
Once determined, the appropriate device classification then needs to find the substantial equivalent device. Substantial Equivalence essentially proves that the current device, compared to a reference device, has the same intended use and technical characteristics or has the same planned application.
The technical variations do not pose any new concerns regarding safety and effectiveness.
Who should do the 510k submission?
- Domestic manufacturers are introducing a medical device to the U.S. market.
- Re-packer or Re-labeler who makes labelling changes or whose operations significantly affect the device.
- Foreign manufacturers/exporters or U.S. representatives of foreign manufacturers /exporters are introducing medical devices to the U.S. market.
When bringing a medical device to market in the United States, based on the risk class and intended use of the device to determine whether to (a) submit a premarket notification, also known as 510k, or (b) petition for premarket approval (PMA) exempted device. While these three terms may sound similar, the amount of time, money, and documentation involved with each are entirely different.
All these complexities necessities the importance of appointing a consultant or agent for various types of FDA 510k submissions.
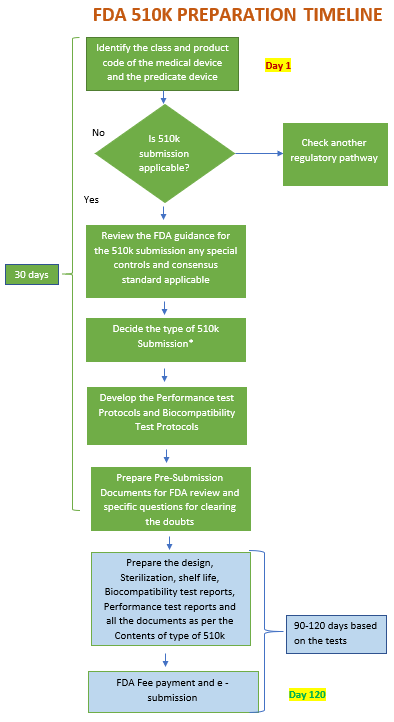
FDA 510k Submission & Approval Timeline
|
PHASE I |
Stages |
Activity |
Responsibility |
Timeline |
|
1 |
Select the Medical Device and models for US FDA Approval |
CLIENT |
20 Days |
|
|
2 |
Identify Predicate Device with the same indication and technology |
CLIENT + MDC |
||
|
3 |
If NOT substantially equivalent, follow the PMA route or substantially equivalent follow the 510k route |
CLIENT + MDC |
||
|
4 |
Appoint MedDeviceCorp as Technical Consultants and US Agent for clearance |
CLIENT |
||
|
PHASE II |
5 |
Identify device Code and Regulation Number along with verification of Predicate Device, indication & technology. |
MDC |
90 Days |
|
6 |
Identify the device Class and guidance document |
MDC |
||
|
7 |
Biological evaluation and test requirement Identification in line with the predicate device |
MDC |
||
|
8 |
Samples send to the Laboratory |
CLIENT |
||
|
9 |
Evaluation of equivalent device compilation |
MDC |
||
|
10 |
Drafting of 510k file in line with available FDA guidance document. |
MDC |
||
|
11 |
Review of Risk analysis, Equivalent device data, Biocompatibility Test / Safety test protocols |
CLIENT + MDC |
||
|
12 |
Review of Labels, User Manual / IFU, Shelf-life records/lifetime calculation, and preclinical study evidence |
CLIENT +MDC |
||
|
13 |
Pre-submission |
CLIENT + MDC |
||
|
PHASE III |
14 |
Compilation by incorporating the pre-submission comments |
MDC |
90 Days |
|
15 |
Compilation of Preclinical and Biocompatibility / Safety testing |
MDC |
||
|
16 |
Compilation and release of the Final Draft |
MDC |
||
|
17 |
Review |
MDC |
||
|
PHASE IV |
18 |
US Agent Appointment |
CLIENT |
20 Days |
|
19 |
Review payment |
CLIENT |
||
|
20 |
Submission in Hard copy and E Copy |
MDC |
||
|
21 |
Receipt of acknowledgement |
CLIENT |
||
|
22 |
Wait for the review comments |
CLIENT |
90 Days | |
|
PHASE V |
23 |
Modify the 510(k) and provide additional supporting documentary evidence as per FDA review comments |
CLIENT + MDC |
60 Days |
|
24 |
Resubmission |
MDC |
10 Days | |
|
25 |
Wait for the review comments or 510k clearance letter |
CLIENT |
90 Days | |
510k Submission & Documentation Fees
(510K Consulting, Preparation, Pre-submission, Final Submission & US Agent)
- US FDA Medical Device Establishment Registration FY 2024 Fees: $7653
- FDA 510k Review Fee FY-2024 (Standard): $21760
- FDA 510k Review Fee FY-2024 (Small Business): $5440
Clients may make the above payments directly to the US FDA
OPTION 1: FDA 510k Complete Package Fees
Payable to MedDeviceCorp [Oct 2024 – Sep 2025]
|
Type of Device |
Guidance Fee |
510(k) (File) preparation |
Submission Fee |
|
Non-invasive |
8000 – 9000 USD |
6000 – 7000 USD |
500 USD |
|
Invasive |
10000 – 11000 USD |
8000 – 9000 USD |
500 USD |
|
Implant |
12000 – 13000 USD |
10000 – 11000 USD |
500 USD |
OPTION 2: FDA 510k Partial Service Fees
Payable to MedDeviceCorp [Oct 2024 – Sep 2025]
|
Activity |
Cost |
Remarks |
|
USFDA 510(k) detailed Review |
6500 – 8500 USD /510(k) |
Device Code, Regulation Number & Predicate device accurate information must be provided by the client. Timeline 30 working days |
|
USFDA 510(k) Quick Review + FDA Pre-Submission * |
6000-7500 USD/510(k) |
Expected timeline 90 to 120 days. US Agent Fees, File Conversion & Stationery / Courier charges included ( No payment needed for FDA) * 510(k) submission can be added to the above service with additional fees of 1500 USD |
|
USFDA 510(k) Pre-Submission |
3500 USD /510(k) |
US Agent Fees, File Conversion & Stationery / Courier charges included Expected timeline 90 days. ( No payment needed for FDA) |
|
USFDA 510(k) Pre-Submission + FDA 510k Submission |
6500 USD / 510(k) |
Expected Pre-Submission timeline 90 days Expected 510(k) Review timeline 120 days FDA Pre-Submission is free and 510(k) Submission FY review charges are applicable US Agent Fees, File Conversion & Stationery / Courier charges included |
Important Information
- Country-specific Government taxes are additional.
- 510k Consultant onsite visit based on customer request. Travelling and Boarding extra invoiced on actual.
- Project cost is divided into 4 instalments based on the progress of the project.
- Method of communication – Email / Telephone / Skype / WhatsApp.
- Language for communication – English.
- Complete documentation by Cloud server.
- 510k preparation timeline 60 to 90 days and Pre-submission approx. 90 days.
- The expected timeline for completing FDA multiple reviews and Clearance is 140 – 180 working days.
Looking for a detailed estimate? Please fill up the Request for Quote and submit it online. Our Experts will study the information submitted and will revert back to you soon.
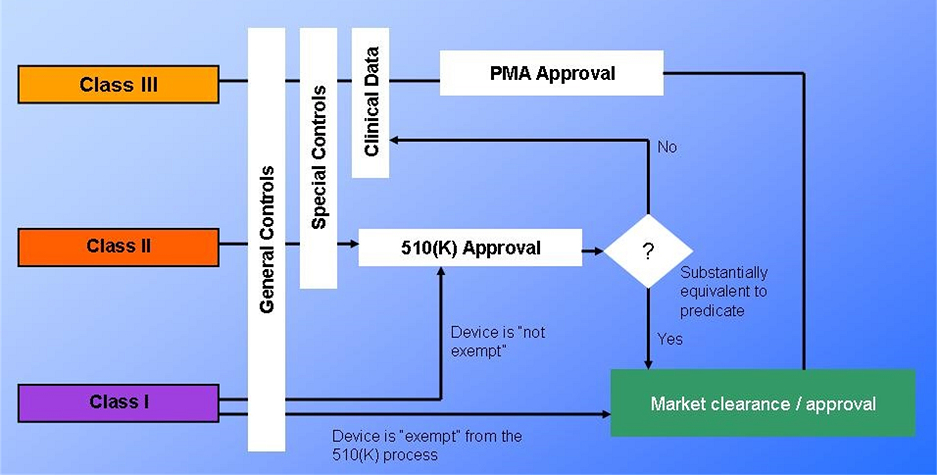
Medical Device Classification
According to the US FDA, the definition of Medical Device varies slightly from MDD. The classification method and criteria also differ. The US FDA has established classifications for approximately 1,700 different generic types of devices and is categorized into 16 medical specialties.
Each of these generic types of devices is assigned to one of three regulatory classes based on the level of control necessary to assure the safety and effectiveness of the device. The three classes and the requirements which apply to them are:
Class I General Controls
-
-
- With Exemptions
- Without Exemptions
-
Class II General Controls and Special Controls
-
-
- With Exemptions
- Without Exemptions
-
Class III General Controls and Premarket Approval
Class 1 or 11 devices if not exempt, you need to select the 510k route for marketing. All devices classified as exempt are subject to the limitations on exemptions. Limitations of device exemptions are covered under 21 CFR 862-892.
A premarket approval application (PMA) will be required for Class III devices unless your device is a pre-amendments device or substantially equivalent to such a device, and PMAs have not been called for. In that case, an FDA 510k will be the route to market.
The classification of a device is determined by its intended usage as well as its indications for use. Furthermore, classification is risk-based, which means that the risk posed by the system to the patient and/or the user is a significant factor in the class to which it is classified.
Class I devices have the lowest risk, while Class III devices have the highest risk. All classes of devices are subject to General Controls, as mentioned above. The Food, Drug, and Cosmetic Act (FD&C Act) establish the minimum standards for all medical devices, including Class I, II, and III.
Find Device Class
The class of device can be identified from the database
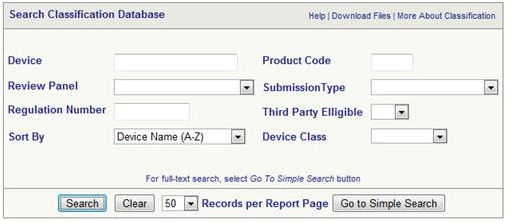
The same can be used to find the following
- To find the class of your device
- To find any exemptions that may exist 510k
- To find the Regulation Number
- To find out whether GMP is applicable or not
- To find out the device description
- To find out 21 CFR Part Number
If you can identify the right product code before approaching us, we will speed up our advice with more accurate information.


Our Social Activities