US FDA Registration For Medical Devices
US FDA Registration process varies depending on the type of product but generally involves an annual or biannual, if commercially available, distribution in the US Market. We are a US FDA registration Consultant supporting worldwide manufacturers & distributors, simplifying a comprehensive and often frustrating and complicated online US FDA Registration Certificate and listing formalities.
FDA (Food and Drug Administration)
The Food and Drug Administration of the United States (US FDA) is responsible for protecting public health by ensuring the safety, efficacy, and security of Food Supply, Drugs and Biological(s), Medical Devices, Cosmetics, Radiation Equipment, and Tobaccos by regulating the manufacturing, marketing, and distribution.
Owners or operators of places of business (also called FDA ESTABLISHMENTS or FACILITIES) that Manufacture, Repack, Relabel, Export, or Import products in the aforementioned industries must REGISTER their facilities with the US FDA. The FDA Registration process varies depending on the type of product but generally involves an annual or biannual, if commercially available, distribution in the US.
TYPES OF PRODUCTS WE COVER
FDA Food Facility Registration
The FDA Food Registration in the United States is effective as of December 12, 2003. The Public Health Security and Bioterrorism Preparedness and Response Act of 2002, also known as the “Bioterrorism Act,” instructs the Department of Health and Human Services’ food regulatory agency. The so-called Food and Drug Administration (US FDA) will take action to protect the public from a terrorist assault on the US food supply, as well as other food-related emergencies.
To comply with the above provisions of the Bioterrorism Act, FDA established the following mandatory regulations:
- All Food Facilities Register with FDA so-called “FDA Registration.”
- Prior information on shipments of imported food to FDA so-called “Prior Notice.”
Manufacturers, distributors, owners, operators, or agents in charge who are responsible for the selling of food for use in the United States must register the facility with the FDA. The FDA is responsible for ensuring that foods sold in the United States are nutritious, wholesome, and properly labeled under the Federal Food, Drug, and Cosmetic Act (FD&C Act) and the Equal Packaging and Labeling Act.
FDA Food Facility Registration can be completed in less than 8 hours. The laws refer to both domestically grown foods and foods imported from other countries.
Types of FDA Inspection & Audit
FDA intends to protect the public from unsafe products; the below four different types of inspections conducted
First Time FDA Food Facility Registration Process
To full fill, the requirement, (a) Food Manufactures, (b) Food Exporters, (c) Exporters with Warehouses must compulsorily register their facility with US FDA.
Our US AGENT & Consulting Service helps to complete the FDA Registration and Certification within a short time.
After completing FDA Food Facility Registration, you can look up FDA registration status from the below link.
Fda Drug Establishment Registration
A drug, in general, is any substance that may alter normal bodily function when administered into the body, either internally or externally, for the treatment, mitigation, or prevention of any disease or condition in a living organism. There is no single, precise description since drug control legislation, government laws, medicine, and colloquial use all have different definitions.
We are FDA regulatory consultants who are fast and cost-effective.
-
- US FDA GMP Consultancy for Pharmaceuticals and API,s
-
- US FDA pre-audit inspection
-
- US FDA post-audit support for closing 483’s
-
- US FDA facility design for the manufacturing of drugs
-
- US FDA establishment registration
-
- US FDA drug listing
-
- US Agent service
Regulatory requirements describe what is necessary for a new drug to be approved for use in patients, further; it requires environment, process controls, documentation, and quality system requirements for the manufacturing of drugs and cosmetics. The Food and Drug Administration in the United States of America is responsible for establishing and monitoring these regulatory standards. The nuances of the rules, as well as their own mechanisms for gaining regulatory approval, make it extremely difficult to obtain and comply with the requirements. Due to the growing costs of meeting various country requirements, an International Conference on Harmonization was created (ICH).
In the United States of America, it is the function of the Food and Drug Administration to establish and monitor these regulatory requirements. The complexities in the laws and their own methods for obtaining regulatory approval make the task of getting and complying with the requirements made very difficult. The rising cost of meeting various country requirements led to the establishment of an International Conference on Harmonization (ICH). The ICH came up with common requirements accepted by all.
National Drug Code (NDC)
National Drug Code (NDC Code)is a ten-digit unique product identifier number for human drugs.
- The first segment (4 or 5 digits) of the NDC Labeler code (NDC Code) identifies the establishment. (manufacturer, packer, labeler, etc. )
- The second segment of the NDC Labeler code identifies the drug (strength, dosage, and formulation)
- The third segment of the NDC Labeler code identifies the package size and package type
The second and third segments of the NDC Code are assigned by the labeler/owner.
NDC code requests to FDA should submit in SPL (structured Product Labelling) format via FDA ESG (electronic submission gateway).
Drug Listing
Domestic and foreign drug manufacturing and Brand owners must list all drugs produced, prepared, propagated, compounded, or processed for commercial distribution in the United States of America along with drug establishment registration. All foreign drug establishment registration must also recognize a US Agent.
FDA Drug Label Review
Drugs must be labeled according to FDA Regulations. We offer Label review services. Our Label review team works in 3 shifts, 24 X 7, and completed more than 2800+ reviews so far across the globe.
The Food and Drug Administration (FDA) is responsible for assuring that foods sold in the United States are safe, wholesome, and properly labeled. … It is the responsibility of the food industry to remain current with the legal requirements for food labeling.
We help manufacturers and Distributors to complete Drug Establishment Registration, NDC Code request to FDA will complete in a matter of 14 working days approximately. We also review the drug label before listing it.
Before being distributed, API and homeopathic drugs should also complete the FDA Drug Listing.
Establishment Registration & Device Listing
For US FDA, Class I was exempted and Class I, II & III 510k cleared FDA Medical Device Registration.
The following type of establishments can register, list and market the medical device in the United States of America:
- Contract Manufacturer
- Contract Sterilizer
- Foreign Exporter
- Initial Distributor
- Manufacturer
- Repackage
- Relabelers
- Remanufacturer
- Preprocessors of Single-Use Devices
- Specification Developer
- U. S. manufacturer of export only devices
The Route of FDA Medical Device Registration
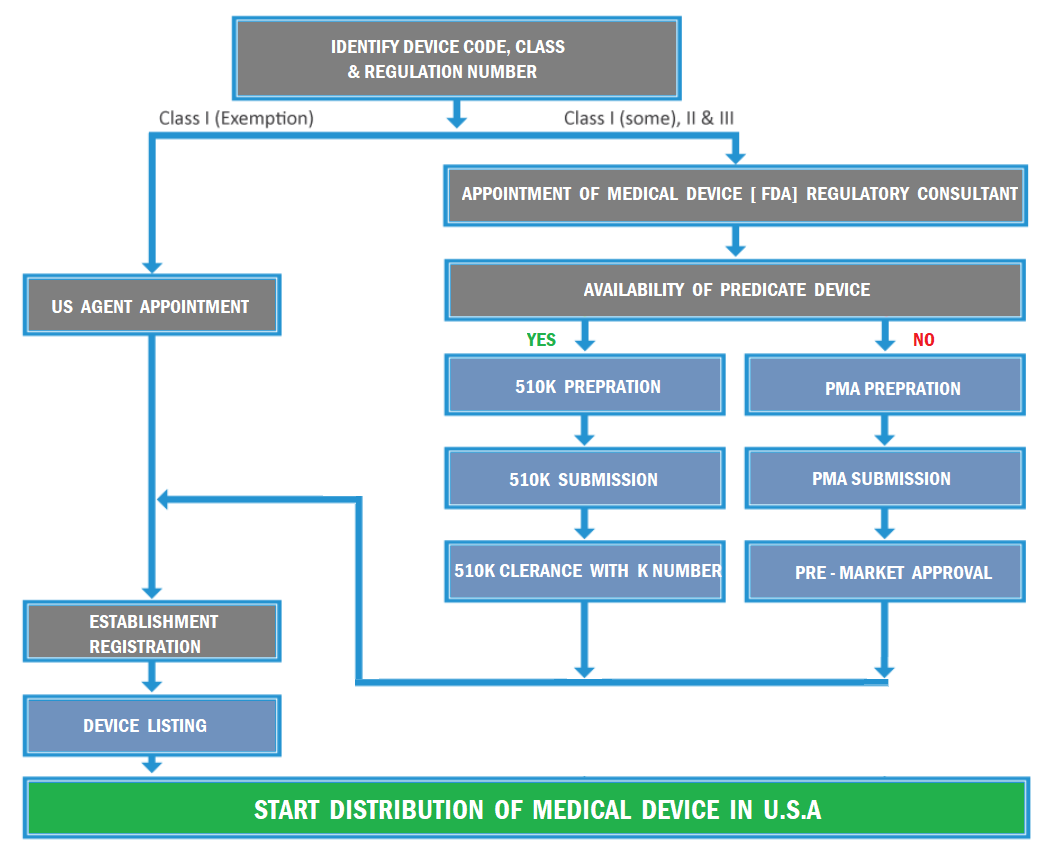
Those involved in the manufacture and sale of FDA medical devices for commercial distribution in the United States must register with the FDA annually. The majority of establishments that are expected to register must also list the devices.
If a device requires premarket approval or notification before being marketed in the U.S., the owner/operator should also submit the FDA premarket submission number/510k. The requirements for FDA Medical Device Registration and listing are based on the type of activity performed at that establishment. All initial importers must review the list of manufacturers of your imported devices. All foreign establishments must review your list of known importers for each of your exported devices. If this information has not been entered previously, it must be entered during the annual registration period to complete registration for the current fiscal year.
Cosmetic Facility Registration (US FDA)
Cosmetics are exempted from FDA premarket approval or mandatory establishment registration or ingredient reporting. The manufacturer’s responsibility is to ensure that their cosmetic products and ingredients are safe and properly labeled, fully compliant with US FDA regulations.
The Voluntary Cosmetic Registration Program (VCRP) is an FDA post-market reporting system for use by manufacturers, packers, and distributors of cosmetic products that are in commercial distribution in the US. There are two parts to the FDA Voluntary Cosmetic Registration program; you may participate in both parts of the program or only one part.
The VCRP applies only to cosmetic products sold in the United States. It does not apply to cosmetic products for professional use only, such as products used in beauty salons, spas, or skincare clinics. It also does not apply to hotel samples or gifts, or cosmetic products you make in your home to sell to your friends.
Cosmetic Facility registration with the US FDA
We help manufacturers and packers interested in registering their establishments with US FDA to sell their products in the US market. Distributors are not allowed as per the law.
FDA assigns a registration number to each manufacturing establishment registered and sends you a receipt.
Cosmetic Product Ingredient Statements (CPIS) Filing with FDA
We help manufacturers, packers, private labelers, or distributors file a statement for each product the firm has entered into commercial distribution in the United States.
FOOD & FOOD PACKAGING MATERIAL TESTING
Test the product as per standard and make sure the safety and quality of the product. Export of food to other countries is increasingly subject to scrutiny, testing to ensure compliance with food safety regulations of the respective countries.
Our labs are accredited and widely accepted by all regulatory authorities. We provide testing for US FDA related submissions and clearances.
|
Testing |
Standard |
Timeline |
| Light Transmittance |
As per Client Requirement |
5 days |
| Dissolvent residue test |
As per Client Requirement |
5 days |
| Microbiological tests |
As per Client Requirement |
15 days |
| Cytotoxicity |
As per Client Requirement |
30 days |
| Penetration of Water Vapour |
As per Client Requirement |
5 days |
| Penetration of Oxygen |
As per Client Requirement |
5 days |
| Heavy Metals |
As per Client Requirement |
15 days |
| Maximum extractable fraction |
As per Client Requirement |
10 days |
| Total Extractive test |
As per Client Requirement |
5 days |
| Water vapour transmission rate |
As per Client Requirement |
5 days |
| Bursting strength |
As per Client Requirement |
5 days |
| Peel strength |
As per Client Requirement |
5 days |
| Pinhole |
As per Client Requirement |
5 days |
| Adhesion |
As per Client Requirement |
5 days |
| Identification of shellac coating (if used) |
As per Client Requirement |
5 days |
| Food Ingredient Identification |
As per Client Requirement |
15 days |
| Stability Studies (1 / 2/ 3/ 4 /5) Years |
Accelerated Conditions |
Depends |
Is It Really FDA Approved? – useful Information
Food Additives & Ingredients – Useful Information


Our Social Activities