FDA 510k Consultants For Medical Devices & IVDs
FDA 510k consultants are highly recommended to assist you with clearance, preparation, timely submission, review, US Agent, and answering FDA review comments. We have highly qualified FDA 510k consultants with technical and scientific knowledge above 10 years.
The process of FDA 510k submission is detailed below.
- Initial GAP assessment of the medical device in the scope of FDA 510k
- Product analysis
- Predicate finding
- Product classification
- Identify FDA guidance for the specific product
- The requirement of performance data study
- Prepare a technical comparison of your medical device to the predicate device(s).
- Product description writing
- Label review and modification
- FDA 510k document preparation (we develop, edit, review 510k compiled the file. We will not allow the client to work on documentation)
- Prepare all 21 sections of the FDA 510k application.
- Coordinate for making payment of FDA 510k submission fees.
- Submit the hard copy and eCopy to the CDRH division within the FDA
- 510k follow-up with FDA officials and will be correspondent for further communications with the FDA.
- Responses to requests for additional information in the shortest time frame.
- For foreign exporters, we act as US Agents for your firm.
- US FDA Agent Immediately communicate with you regarding all information received from the FDA following the FDA submission and assist in addressing requests for additional information, if applicable.
FDA will not perform any ONSITE inspection as part of 510k clearance. The manufacturer should implement FDA Quality System as per 21 CFR 820 and be ready to face inspection by FDA auditors at any time after marketing the devices in the USA.
FDA 510k Consultants Roles and Responsibilities
The following are the significant steps involved in the preparation and submission of FDA 510k.
- Step 1: Identify the device regulation number and device code.
- Step 2: Discuss and debate with the client to identify the predicate /equivalent 510k cleared device.
- Step 3: Identify 510k type a. Traditional 510k b. Abbreviated FDA 510k c. Special FDA 510k.
- Step 4: FDA 510k Preparation, along with preclinical studies and External testing.
- Step 5: 510(k) Pre-submission to FDA.
- Step 6: 510k updation as per FDA review comments.
- Step 7: US Agent appointment and FDA review Fee payment.
- Step 8: US FDA 510(k) submission ( Hard copy & E copy).
- Step 9: Communicate and follow up with the FDA (on behalf of the client).
- Step 10: FDA 510k modification and resubmission as per FDA review comment.
- Step 11: Wait for additional review comments, if any, till receipt of the 510(k) number.
- Step 12: Establishment Registration with 510(k) number and list the 510(k) cleared device.
- Step 13: Initiate sales and marketing of the device in the USA.
Detailed FDA 510k Submission Process
- Appoint FDA 510k Consultants: The consultants or consulting team should have extensive knowledge and experience with 510k submissions for medical devices. The consultants should be in charge of your submissions and communications until you receive your K number.
- Determine whether your product (a) is a medical device or (b) a radiation-emitting product: Once it is identified, proceed with compiling the FDA regulatory requirements required for marketing the product in the US.
- Classify the Medical Device: This will help us make sure the 510k route can be used to market along with the device code submission procedure and other applicable compliance issues.
- Implement 21 CFR 820 if Applicable: If the product is not exempted from GMP, you need to implement GMP in the manufacturing location. This is not a requirement for 510(k) submission, but all firms registered should be prepared to face Inspection; if failed FDA will issue a warning letter to the manufacturer, and you will be forced to withdraw products from the market.
- Test the product/Equipment: For preparing FDA notifications, test reports and datasheets are essential. The applicable standards have to be identified, and testing has to be done from accredited laboratories.
- Review the Test Report: Review of test reports to be carried out based on the standards and acceptance criteria. Any errors in the report cannot be used in the 510(k) notification.
- Appoint US Agent: Appoint a US Agent to assist the FDA in communications with the foreign establishment, answering questions concerning the foreign establishment’s devices to be imported into the United States.
- Prepare 510k Notification and Submit: Prepare 510(k) notification with the help of a 510k consultants, and submit to the FDA directly or through FDA-approved third-party agencies such as UL, Intertek, and BSI with the help of a Consultant and US Agent.
- Register the establishment: The manufacturer has to register the establishment involved in the production and distribution of medical devices intended for use in the United States are required to register annually with the FDA under section 510(g) of the act. An owner or operator of an establishment located in any of the states in the US as defined in section 201(a)(1) of the act shall register its name, and places of business and list the devices if interstate trading is done.
- List the device with the FDA: Once the establishment registration is done, the products intended to be sold in the US have to be listed under registered establishments. If a device requires premarket approval or notification before being marketed in the U.S., then the owner/operator should also submit the FDA premarket submission number.
Medical Device Classification
According to the US FDA, the definition of Medical Device varies slightly from MDD. The classification method and criteria also differ.
The US FDA has established classifications for approximately 1,700 different generic types of devices and is categorized into 16 medical specialties.
Each of these generic types of devices is assigned to one of three regulatory classes based on the level of control necessary to assure the safety and effectiveness of the device. The three classes and the requirements which apply to them are:
Class I General Controls
- With Exemptions
- Without Exemptions
Class II General Controls and Special Controls
- With Exemptions
- Without Exemptions
Class III General Controls and Premarket Approval
Class 1 or 11 devices if not exempt, you need to select the 510k route for marketing. All devices classified as exempt are subject to the limitations on exemptions. Limitations of device exemptions are covered under 21 CFR 862-892.
A premarket approval application (PMA) will be required for Class III devices unless your device is a pre-amendments device or substantially equivalent to such a device, and PMAs have not been called for. In that case, an FDA 510k will be the route to market.
The classification of a device is determined by its intended usage as well as its indications for use. Furthermore, classification is risk-based, which means that the risk posed by the system to the patient and/or the user is a significant factor in the class to which it is classified.
Class I devices have the lowest risk, while Class III devices have the highest risk. All classes of devices are subject to General Controls, as mentioned above. The Food, Drug, and Cosmetic Act (FD&C Act) establish the minimum standards for all medical devices, including Class I, II, and III.
Find Device Class
The class of device can be identified from the FDA database
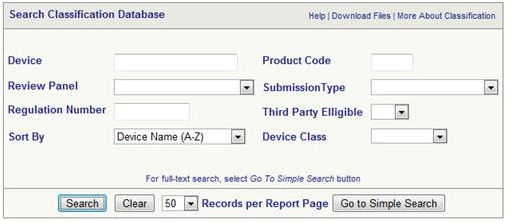
The same can be used to find the following
- To find the class of your device
- To find any exemptions that may exist 510k
- To find the Regulation Number
- To find out whether GMP is applicable or not
- To find out the device description
- To find out 21 CFR Part Number
If you can identify the right product code before approaching us, we will speed up our advice with more accurate information.
The Advantages of Working with FDA 510k Consultants
- Our technical FDA 510k consultants have previous experience in helping improve the quality of 510k documents and reduce the review and communication time.
- 26+ full-time regulatory consultants. Separate consultants are involved in the development of individual modules/annexes.
- One-stop solution for 510k Consulting, Testing and US Agent Services.
- Payments based on deliverables (Stage-wise payment). Payment options in INR, USD, and EUR.
- Offices in Germany, India & the USA. The average 510k preparation timeline is 90 days.
- Experience counts more than anything!
Fill The Below Form and we will get back to you.


Our Social Activities